In the late 1960s, James Allison became enthralled by T cells in an undergraduate classroom at the University of Texas at Austin. T cells, newly discovered at time, are immune cells that travel through the body, hunting for invaders or abnormal cells that might cause harm. When they find something suspicious, they multiply and eliminate the threat.
Curious, Allison sought out his professor after class for more details: how do these cells recognize pathogens? What makes them decide to proliferate? What drives them to attack invaders — and what holds them back? He was disappointed to learn that his professor had no answers; scientists knew little about these cells and their astonishing abilities.

Allison at a MD Anderson Research Facility in Smithville, Texas
[Courtesy of University of Texas MD Anderson Cancer Center]
“I never considered myself a cancer biologist,” Allison says. “I really was just trying to figure out how T cells worked.” While working out the details of T-cell behavior, Allison also learned how they interact with tumors, enabling him to harness them for new cancer treatment strategies that have already saved countless lives.
Finding the Holy Grail
When Allison accepted his first faculty position in 1977 at MD Anderson Cancer Center, he decided to tackle an ambitious project: “The holy grail of immunology of that time was the antigen receptor — the thing that T cells use to recognize stuff that ought not to be there,” he recalls. “I figured out a strategy using biochemistry and making some assumptions about what [the receptor] should look like and then succeeded in working out something that had all those properties.”
Allison and his colleagues published that they had found the long-sought-for T-cell antigen receptor in 1982 and waited for a response from the scientific community. The field at the time was highly competitive, and initially, researchers viewed the data skeptically.
Then one day, Allison received a call from one of the leading T-cell biologists in the world, Philippa Marrack from the National Jewish Hospital and Research Center in Denver, Colorado, who asked him to present his data at an upcoming conference. “To tell you the truth, I was scared!” says Allison. “I didn’t have any kind of name recognition or anything.” But he accepted the invitation and presented his work.
Unbeknownst to Allison at the time, Marrack had already confirmed his results. Following Allison’s talk, she presented research from her own laboratory verifying that Allison had in fact identified the T-cell receptor. “Everybody accepted it at that point,” he says.
The T-cell receptor is a small protein on the surface of the cell that recognizes foreign proteins on other cells. When it meets one of these foreign proteins, it triggers a chain reaction that directs the immune response. Essentially, Allison had identified the T-cell start button and had simultaneously opened the door for scientists to begin to tease out exactly how T cells coordinate their assault on pathogens.

Allison at a MD Anderson Research Facility in Smithville, Texas
[Courtesy of University of Texas MD Anderson Cancer Center]
Disabling the Brakes
While the CTLA-4 debate transpired, Allison dabbled in a side project in his lab. “I always sort of had cancer in the back of my mind,” says Allison, who lost his mother, two uncles, and a brother to cancer.
Since the late 1800s, scientists had aspired to use the immune system to thwart cancer, but after a widespread series of failures, the idea had fallen out of favor. “People were making a lot of arguments about why the immune system would never be good at treating cancer,” Allison recalls. One prevailing view was that cancer arose from the body’s own cells, so unlike a viral infection, there was nothing foreign in a tumor to incite the immune system. “I always thought that was silly. After all, we can reject whole [transplanted] livers and entire organs — kidneys — because of a single molecule mismatch.”
Allison’s team initially altered tumors to force the immune system to recognize them. Promising early results in mice confirmed that the immune system could identify and target cancer cells, but it didn’t look feasible to implement this approach clinically. They next tried a vaccination approach by injecting tumor proteins into mice to initiate an immune response, but they encountered a lot of technical problems and eventually abandoned the project.
In the midst of these struggles, Allison realized that T cells activate when they encounter tumor cells, but at the same time, CTLA-4 begins to block their response. “It’s just sort of a clock; it turns the T cells off after a time,” Allison explains. A tumor is large compared with other pathogens or infected cells the immune system encounters, so he reasoned that T cells simply don’t have enough time to conquer the tumor before they are shut off again by CTLA-4 activity.
“I thought, well, let’s just disable the brakes with an antibody against CTLA-4 and keep [T cells] going as long as we need to so that they can take the tumor out,” Allison says. He and his team did exactly that. The process they developed, now referred to as immune checkpoint inhibition, essentially deactivates the T-cell shut-off switch, leaving T cells active long enough to fight the tumor.
“And it worked! To my surprise, it worked amazingly well. In most tumor models we looked at, it would just melt the tumors completely.”
The team tested their approach on spontaneous tumors and clinically induced tumors. They treated multiple mouse strains to be sure the response wasn’t specific to a certain type of mouse, and they explored cancers in a wide variety of tissues. “It worked so broadly that I said this has got to work in humans,” Allison reports.
Allison wrote a patent for his checkpoint inhibitor therapy strategy and started searching for a biotechnology company to develop the drug.

Allison (left) with fellow awardees Evelyn Witkin and Stephen Elledge at the 2015 Lasker Luncheon
Unchecking T Cells
Allison eventually connected with a company called Medarex that was willing to take a chance on blocking CTLA-4 to allow T cells enough time to conquer a tumor. Medarex developed the treatment and started a phase I trial in 2001.
“Usually, phase I is just to look for safety and dose escalation,” Allison explains. “But it worked in three people with melanoma. At that time, there was no drug at all that had ever extended life for melanoma patients.”
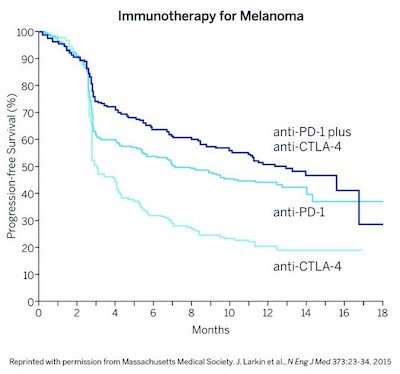
Anti-CTLA-4, Anti-PD-1, and the two together extend the length of time that patients live without worsening. The combination therapy works better than either agent alone.
[Courtesy of Cassio Lynm]
Recent studies following up on the early trials indicated that 22% of patients with advanced melanoma — a disease that previously claimed the lives of 50% of patients within a year of diagnosis — lived at least 10 years after treatment. “I think that with some tweaks here and there, we can get that [percentage] up higher,” Allison said.
In collaboration with his research partner and spouse, Padmanee Sharma at MD Anderson Cancer Center, Allison is now studying exactly what happens in T cells when checkpoints are blocked and what downstream repercussions that has on other cells in the body. The team is also collecting tumor tissues and blood from patients with cancer treated with a wide array of therapies to find out what is happening on a cellular level in each case. The immediate goal is to collect enough data for clinicians to make informed choices about combining therapies. In the long term, they hope that these studies will shed light on why some patients don’t respond to treatment and reveal ways to modify the treatment to achieve the best outcome for each individual.
Within a few years, Allison expects that checkpoint inhibitor therapy will succeed in the majority of patients with cancers where the treatment currently performs well, not in only 22%. And he anticipates that new research and modifications to existing approaches will increase the number of cancer types that yield to this groundbreaking therapy.
“We’re not going to cure all of cancer; that’s absurd,” he says. “I think what the future holds is that immunology is going to be a component of all treatments.”
by Kristie Nybo
