Douglas R. Lowy
National Cancer Institute

John T. Schiller
National Cancer Institute
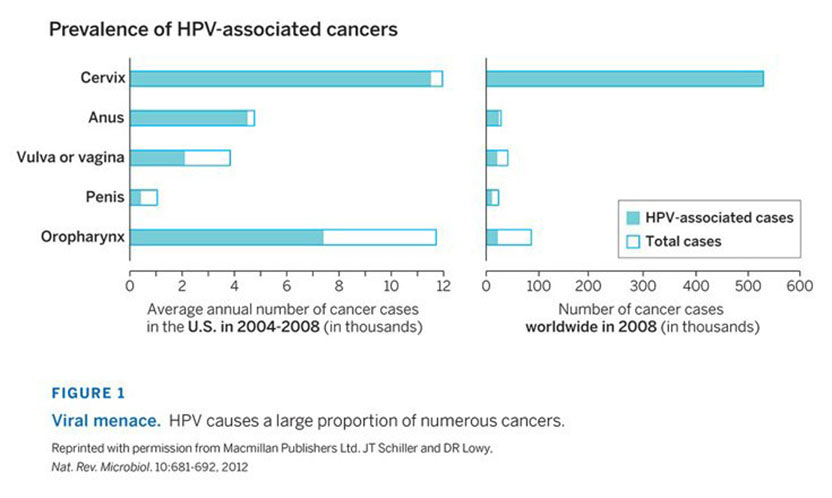
The 2017 Lasker~DeBakey Clinical Medical Research Award honors two scientists whose technological advances enabled the development of human papillomavirus (HPV) vaccines, which prevent cervical cancer and other tumors. Douglas R. Lowy and John T. Schiller (both from the National Cancer Institute) took a bold but calculated approach toward a major public-health problem whose solution required them to vault formidable hurdles. They devised a blueprint for several safe and effective vaccines that promise to slash the incidence of cervical cancer and mortality, the fourth most common cancer among women worldwide, as well as other malignancies and disorders that arise from human papillomaviruses.
Award presentation by Craig Thompson
Today we honor two exceptional cancer biologists from the National Cancer Institute for their foundational studies that enabled the development of a vaccine to prevent cancer.
Cancer is the disease that people throughout the world fear most for themselves and their loved ones.
Every day we hear of promising new treatments for cancer. Recent winners of the Lasker Clinical Award have helped pave the way for important new treatments for cancer. The discovery of Gleevec for the treatment of chronic myelogenous leukemia put precision medicine on the map and won Brian Druker, Nick Lydon, and Charles Sawyers a Lasker for their work that turned a fatal cancer into a chronic disease. In 2015, Jim Allison was recognized for pioneering studies showing that the immune system could be harnessed to fight cancer. His work launched the exploding array of cancer therapies that seek to augment the immune system rather than damage cancer cells. Together, precision medicine and immuno-oncology offer great hope for cancer patients.
Acceptance remarks
Acceptance remarks, 2017 Lasker Awards Ceremony
It is of course gratifying to be receiving a Lasker Award, but the award is really a tribute to the field of HPV research and to our many colleagues who have contributed to it. More generally, the award highlights the importance of the HPV vaccine as an advance in disease prevention that can overcome the worldwide scourge of cervical cancer and other HPV-associated cancers.
When many people think about recent research achievements in cancer and other diseases, the focus is usually on advances in the treatment of established disease. This attention reflects the drama, satisfaction, and relief that come from a new treatment that can heal sick people whose condition would otherwise not have improved.
Much less attention is generally given to advances that can prevent the development of disease, although these advances may often benefit substantially more people than many advances in treatment. At the risk of oversimplification, I see disease prevention as containing an inherent paradox. On the one hand, the great strength of disease prevention lies in the fact that there are no grateful patients. On the other hand, the great limitation of disease prevention lies in the fact that there are no grateful patients. In both situations, we don’t need to worry about contracting the disease.
For biotech and pharma, the financial incentives for developing interventions that prevent disease usually pale by comparison with those that come from developing interventions that treat established disease. Private philanthropy also tends to focus on treatment. Therefore, the development of most interventions that prevent disease depend on investment by the public sector, just as basic research and research on the causes of disease depend almost entirely on long-term, public sector support.
Development of the HPV vaccine was made possible by sustained public investment in biomedical research by many countries. In the United States, the vast majority of this funding comes from the NIH, which has benefited from strong, bipartisan support from elected officials. Public investment in basic research and in technology development led to identifying HPV infection as the main cause of cervical cancer as well as several other types of cancer. Public investment in disease pathogenesis led to understanding how HPV infection can lead to cancer. And public investment supported the translation of this new knowledge into the technology that enabled the HPV vaccine.
It was at this point that Merck, Medimmune, and GlaxoSmithKline began to develop commercial versions of the vaccine. The vaccine manufacturers took considerable risk, as prior to the HPV vaccine, there was no vaccine that could prevent benign and malignant disease that develops at the initial site of infection. The patient volunteers who participated in the trials also played an indispensable role. Fortunately for everyone, the ability of the commercial vaccines to prevent disease caused by HPV has exceeded even our most optimistic expectations, while also highlighting the value of public-private partnerships in health and disease. Amazingly, eliminating cervical cancer and other HPV-associated cancers as a major public health problem is now a realistic goal.
Acceptance remarks, 2017 Lasker Awards Ceremony
Receiving a Lasker award has prompted me to think a bit more about an individual’s role in moving biomedical research forward. Awards tend to focus on individual scientists, or small group of scientists, whose heads are seen to rise out of the broad stream of the biomedical research enterprise. The question arises “Are the scientists who get these awards the key individuals who channel the stream or is it the stream itself that is primarily responsible for moving the individual scientist forward, such that the those that move the fastest are simply better able to stay in the main flow and avoid bumping into the rocks?”
Although it may seem a bit contrary in this context, I would vote for the stream making the more important contribution. In my estimation, the accumulation of technical and conceptual knowledge in a certain field can combine to make even paradigm shifting discoveries inevitable. For example, does anyone here think that if Harold Varmus and Mike Bishop had become astrophysicists rather than molecular biologists that oncogenes would not have been discovered? Proto-oncogenes are too fundamental to biology to have gone undiscovered. . This is not to diminish the cleverness of their experimental approach, the importance of their discoveries, or the appropriateness of their receiving the Lasker and Nobel prizes. It is my belief that, with rare exception, scientists that rise above their peers do so primarily in recognizing and exploiting the opportunities presented by the current state of the art earlier and faster than others. They function primarily in speeding up the clock of what otherwise would have occurred at a slower pace.
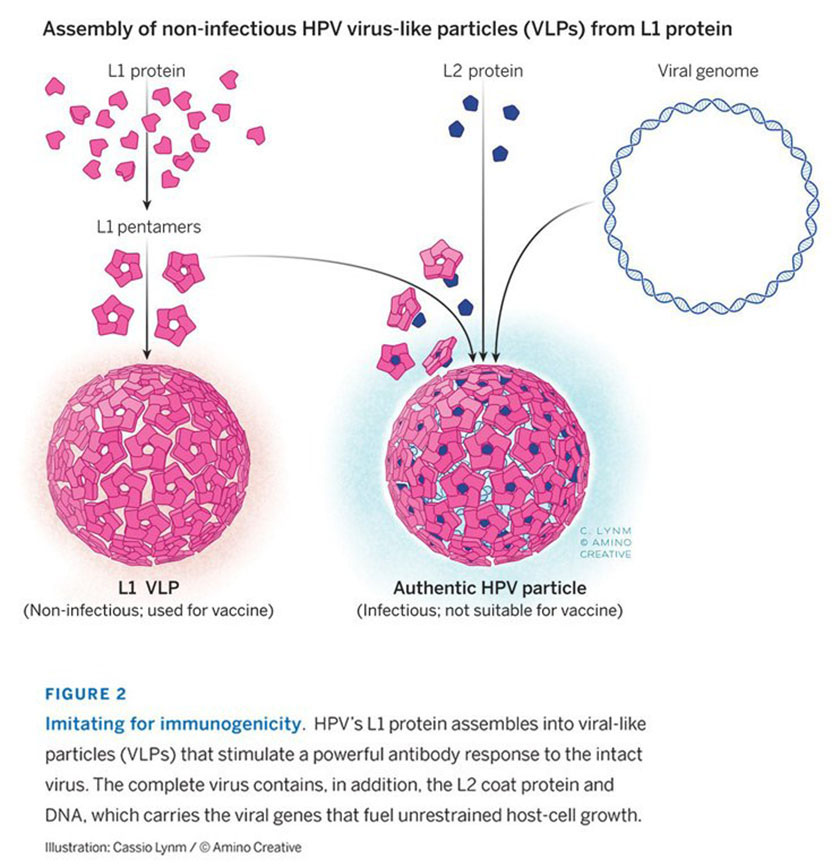
Our own work on HPV vaccines is a good illustration of how prior fundamental accomplishments are the essential precursors to a major advance. For example, without the advent of molecular cloning, the 10 or so HPV types that cause cancer, amongst 100s that don’t, would not have been identified and characterized. Without the development of protein expression systems, we could not have produced the vaccine. Without gene amplification, we would not have had the sensitive assay required for monitoring HPV infection in epidemiological studies that established cancer causation and in the pivotal clinical trials that led to vaccine licensure. None of these critical advances were made with the virus-like particle vaccine in mind. Nevertheless, the fact that 360 copies of a single HPV virion protein can self-assemble into a particle that is incredibly effective at inducing antibodies that block virus infection is such a robust biological phenomenon that it was destined to be discovered given the state of the art at the time.
I would like to leave you with two thoughts about acknowledging the importance of the enterprise over the individual. First, it emphasizes the need for a broad-based approach to funding of both basic and translational research. We can’t pick the winners in advance. And second, relegating the importance of the individual is incredibly comforting in that it implies that major advances in human heath are essentially inevitable if proper resources are devoted to the biomedical research enterprise. So, let’s celebrate the enterprise, as well as the individuals, today.
2016 Clinical Award video
Video Credit: Flora Lichtman