
John B. Glen
AstraZeneca (Retired)
The 2018 Lasker~DeBakey Clinical Medical Research Award honors John B. (Iain) Glen (retired from AstraZeneca), who discovered and developed propofol, a chemical whose rapid action and freedom from residual effects have made it the most widely used agent for induction of anesthesia in patients throughout the world. In 2016, the World Health Organization deemed propofol an “essential medicine” and at the time of that decision, more than 190 million people had received the drug.
Anesthetic aim
In 1972, Glen joined Imperial Chemistry Industries (ICI; through mergers, ICI eventually became AstraZeneca) to help find new short-acting intravenous anesthetics. Eventually he took charge of the enterprise. The type of drug he sought—an induction agent—is used to sedate people so they can then tolerate inhaled anesthetics that maintain unconsciousness for long procedures. Administration of these gases through a mask can cause discomfort, and some of them can provoke an initial feeling of suffocation.
The gold standard induction agent at the time was called thiopentone, which induced anesthesia quickly, but had limitations. Most prominent among them, it builds up in the body, so repeated use during surgery would cause patients to remain unconscious long afterward. Glen’s team wanted to find a medication that possesses the anesthetic power of thiopentone, but allows rapid recovery. In addition to serving as a new induction agent, such a drug might maintain sedation and thus provide an injected alternative to inhaled anesthetics. The group also aimed to reduce common unpleasant after-effects of anesthesia such as nausea and vomiting.
Glen and the ICI team not only had to identify an active ingredient, they also had to find a delivery vehicle for it. This challenge posed a formidable barrier and almost toppled the project at several junctures. To gain access to the brain, anesthetics must be lipid soluble, but to achieve compatibility with blood, they must be presented in an aqueous solution for injection. Soon after Glen’s arrival at ICI, a different anesthetic entered the market; it was dissolved in an ethoxylated castor oil preparation that allowed an aqueous formulation to be achieved. The application of this material with a clinically useful anesthetic presented the possibility of testing a bank of previously synthesized lipid-soluble chemicals that ICI had stored in its compound collection.
Awakening a promising candidate
ICI chemist Roger James chose a number of compounds from the collection, and Glen began screening them in mice. In 1973, he demonstrated that one of these chemicals, propofol (chemical name, 2, 6-diisopropylphenol; trade name, Diprivan®), showed anesthetic promise in a battery of animal tests. In mice, it was 1.8 times as strong as thiopentone—and it matched standard agents in producing a rapid onset of effect. It did not cause the muscle twitching and tremors that plagued many anesthetics, and it could be combined safely with a variety of other drugs typically used for sedation.
Crucially, the compound did not accumulate in the body, even after multiple injections. Glen gave doses that induced short periods of sleep and repeated them each time the animals regained consciousness. The first injection of propofol or thiopentone knocked them out for four-to-five minutes. Ten doses of propofol lengthened that time only slightly—to about 15 minutes. In contrast, four doses of thiopentone rendered the animals unconscious for more than three hours. Subsequent tests of balance showed that the mice recovered quickly from propofol, without the significant hangover effect observed with thiopentone treatment.
Dissolving the problem
ICI launched clinical trials and gathered data on more than 1000 patients. Propofol delivered its anesthetic effects smoothly and safely, and people recovered quickly, with minimal grogginess, nausea, or other lingering unpleasantness.
Despite these positive results, the delivery substance triggered a life-threatening reaction in several individuals, and the trials were halted. The formulation challenges and other setbacks—including dosage issues and pain on injection in some circumstances—were dampening enthusiasm at ICI for the drug. Glen continued to champion the program within the company, and he kept it alive.
The company’s scientists had long wondered whether they could design an emulsion—a suspension of tiny oil droplets dispersed in a liquid—to carry propofol, but technology for producing such a material initially failed to produce sufficiently small particles. Improved emulsion-manufacturing methods allowed Glen, in collaboration with ICI pharmaceutical chemists, to identify constituents with acceptable biological and pharmacological attributes. This project culminated in a soybean oil-based emulsion formulation in which propofol bestowed its anesthetic benefits on animals without serious side effects.
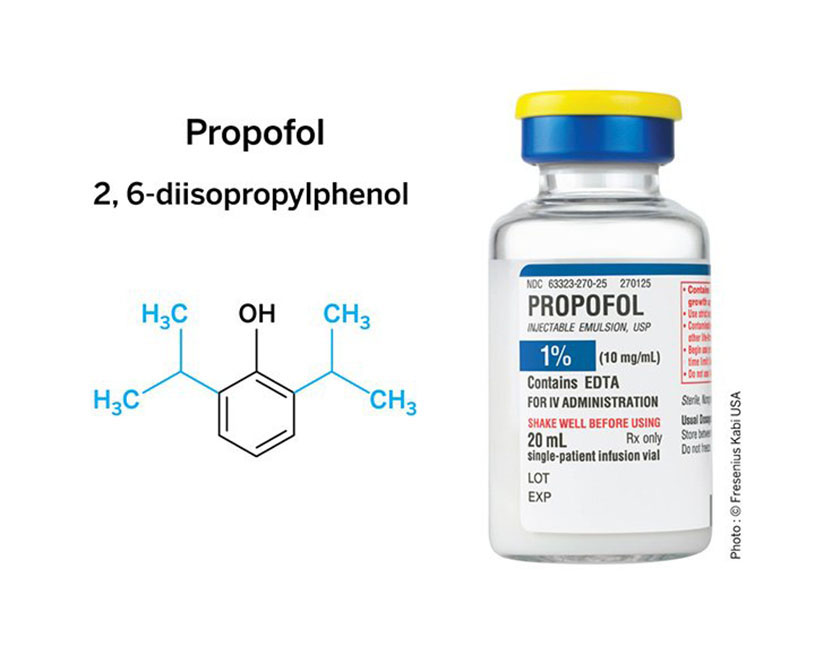
Potent anesthetic: The isopropyl side groups (blue) of propofol’s chemical structure (left) are essential for the anesthetic activity and advantageous side effect profile of the drug. The soybean-derived formulation is a white liquid (right), which has inspired its nickname, Milk of Amnesia.
A global success
The soybean emulsion formulation performed well in people. Results from almost 1500 individuals treated with this version of propofol reflected the safety and efficacy findings obtained previously with the agent—minus the life-threatening reaction. In 1986, the drug received regulatory approval in the UK. Thirteen years had elapsed between its discovery and launch. US FDA approval followed in 1989, and it is now approved in more than 90 countries.
Additional clinical trials led to tremendous expansion of propofol’s applications—in children, for example, and for “maintenance” anesthesia in surgery and critical care settings. That long-term use has proved especially valuable in intensive care units, where it can keep people from fighting mechanical ventilation. The drug can relax an individual who then receives a local painkiller—or one who is undergoing a procedure such as a colonoscopy or bronchoscopy. Because recovery from propofol is rapid, it is especially well suited for outpatient clinics. People can go home clearheaded and free of wooziness soon after their procedures, which increases willingness to undergo ambulatory surgery.
Propofol’s use in maintenance anesthesia was initially limited because giving repeated injections was unattractive, and suitable equipment for continuous administration was not available. In particular, existing pumps could not deliver the medicine rapidly enough. Glen established a collaboration with a commercial manufacturer, Ohmeda Company, and the resulting apparatus has served as a prototype for a new generation of devices.
Subsequently, he advanced deployment of a technology eventually known as target-controlled infusion for propofol. In real time, a computer, programmed with a pharmacokinetic model that describes how the body handles the drug, calculates the amount needed to achieve a particular concentration in the blood or brain, and adjusts infusion rates accordingly. This method produces more predictable outcomes than can be achieved with manual oversight alone and facilitates subtle regulation of anesthetic depth. Glen persuaded his company to launch a target-controlled infusion program and the resulting system is now available in most countries, although not the US. In some areas, it is used extensively to deliver propofol for prolonged periods.
Through his vision and persistence over more than twenty years, Glen ushered into the world’s medical arsenal a powerful and versatile anesthetic that acts quickly and produces minimal side effects. Clinicians routinely deploy it in operating rooms, intensive care units, emergency departments, and outpatient clinics—and it has dramatically eased and expanded ambulatory procedures. Propofol is now the standard intravenous anesthetic induction agent in the US and the world, and it has made a vast range of operations and medical tests comfortable and acceptable for people across the globe.
by Evelyn Strauss
Key publications of John Baird Glen
Glen, J.B., Davies, G.E., Thompson, D.S., Scarth, S.C., and Thompson, A.V. (1979). An animal model for the investigation of adverse responses to i.v. anaesthetic agents and their solvents. Br. J. Anaesth. 51, 819-827.
James, R., and Glen, J.B. (1980). Synthesis, biological evaluation, and preliminary structure-activity considerations of a series of alkylphenols as intravenous anesthetic agents. J. Med. Chem. 23, 1350-1357.
Glen, J.B. (1980). Animal studies of the anaesthetic activity of ICI 35868. Br. J. Anaesth. 52, 731-742.
Glen, J.B., and Hunter, S.C. (1984). Pharmacology of an emulsion formulation of ICI35868. British J. Anaesth. 56, 617-626.
Stark, R.D., Binks, S.M., Dutka, V.N., O’Connor, K.M., Amstein, M.J., and Glen, J.B. (1985). A review of the safety and tolerance of propofol (‘Diprivan’). Postgrad. Med. J. 3,152-156.
Coetzee, J.F., Glen, J.B., Wium, C.A., and Boshoff, L. (1995). Pharmacokinetic model selection for target controlled infusions of propofol. Anesthesiology. 82, 1328-1345.
Glen, J.B. (2017). The development and regulation of commercial devices for the administration of drugs by target controlled infusion, Chapter 2. In Total Intravenous Anesthesia and Target Controlled Infusions: A Comprehensive Global Anthology. Edited by A.R. Absalom, and K.P. Mason, Springer International Publishing AG, Cham, Switzerland, pp. 9-29.

What Makes a Piece of Art or Science a Masterpiece?
Critics of art and philosophers of science have long wrestled with the question of what elevates a piece of art or a set of experiments to masterpiece status.
Award presentation by Lucy Shapiro
In 1846, Henry Jacob Bigelow introduced ether as an anesthetic for surgical patients and changed the face of medicine. 130 years later, beginning in the 1970s, Iain Glen guided the transformation of 2,6 diisopropylphenol from a chemical solvent to the most transformative anesthetic since ether.
Why has this anesthetic been transformative?
- Fast onset;
- Rapidly metabolized and allows very rapid awakening with a clear head;
- Significantly reducing nausea and vomiting;
- Of particular importance is the role that propofol has had in the safe use of imaging studies in young children that requires prolonged immobility for adequate imaging;
- Accelerated the growth of ambulatory surgery. I would wager that most people sitting in this room have benefited from an intravenous infusion of propofol for procedures such as colonoscopy and cataract surgery.
- Adoption of propofol for diagnostic & therapeutic radiological procedures allowed the development of breakthrough imaging technologies;
- Allowed a breakthrough in head & neck surgery because intravenous administration eliminated blocking airway passage by the use of inhaled anesthetics;
- Radically changed the maintenance of anesthesia because it could be infused continuously.
The ubiquitous use of propofol and its benefits to hundreds of millions of patients, a landmark achievement, is due to Glen’s persistence and perseverance in bringing propofol to fruition over many years, despite substantial skepticism at each step.
Iain Glen’s journey from earning a diploma in Veterinary Anesthesia from Glasgow University in 1968, to establishing a transformative anesthetic is a remarkable story of vision, tenacity, and grit by a Scotsman raised and educated in the west regions of Scotland.
As a young vet student, he devised methods to assess new anesthetic agents in animals.
Then, beginning in 1972, as a Research Biologist in Anesthesia at ICI (Imperial Chemical Industries), Glen systematically screened chemical libraries in animals to identify a novel lipophilic chemical for use as an intravenous anesthetic.
Glen reported the identification and chemical synthesis of propofol and presented animal studies verifying its effectiveness. In collaboration with a chemist at ICI, Roger James, Glen described the synthesis and biological evaluation of a large series of alkyl-substituted phenols, one of which would become the active ingredient in propofol.
Propofol was poorly soluble in water and consequently was combined in a castor oil-based formulation.
In 1978, large scale clinical trials of propofol were initiated in the UK, the Netherlands, and Germany.
However, in 1980 the clinical trials were halted due to a small number of adverse reactions (anaphalaxis), and ICI called a halt to any trials or further work on Propofol. This is where, in the first of two decisive moves, Glen stubbornly refused to go along with the corporate “wisdom.”
Glen said not so fast. He showed that the castor oil solvent was responsible for the adverse reactions, not the active ingredient, propofol.
Glen pushed ICI not to discontinue work on propofol, but to let him develop alternative formulations to be used in preclinical models. The fourth formulation, published in 1984, was an emulsion that was both safe and effective. This formulation retained propofol properties and was well tolerated in animal models.
In 1983, Glen then made the rare move from the lab to clinical development, where he oversaw the initiation of clinical trials with the new formulation in Europe. By then, Glen had completed his PhD in pharmacology from Glasgow University for “studies on the pharmacology of intravenous agents.”
The first European approval of propofol was in 1986, in the US, in 1989, and propofol achieved worldwide acceptance for the initiation of anesthesia. It completely replaced sodium thiopental, the then intravenous anesthetic induction agent of choice. As of today, thiopental has virtually disappeared from anesthetic practice, with the consequent huge improvement in patient mobility and mortality.
However, after injection of propofol to initiate anesthesia, the maintenance of anesthesia continued to be an inhalation agent.
Glen then pursued the second phase of his vision for propofol – the maintenance of anesthesia by infusion systems. Owing to its favorable pharmacokinetic properties, it was clear to Glen that propofol could be used for the long-term maintenance of anesthesia without the secondary use of inhaled anesthetics. To do this, Glen’s goal was to develop a hybrid between a drug and a computer software system for the continued administration of the drug. ICI was not inclined to pursue this route. So, undeterred, Glen hosted two international meetings that provided the expertise to generate software for continuous administration of intravenous propofol (by this time, ICI had morphed into Zeneca, that would later become Astra Zeneca). In 1993, Zeneca agreed to support the commercial development of a practical and safe Target Controlled Infusion system, which was launched in the UK in 1996. It is now available in 90 countries and millions of patients each year receive propofol anesthesia by continuous infusion. (parenthetically, it is still in the approval process in the US).
Iain Glen’s vision, creativity and persistent stewardship of the chemical screening to identify propofol, the design of the critical formulation for anesthetic delivery, the design of the clinical trials, and the clinical development and approval for continuous intravenous drug delivery, has transformed the hospital experience for hundreds of millions of patients.
Acceptance remarks by John B. Glen
It is indeed a great honour to accept this award, and to be here today among so many brilliant scientists. Science can be a lonely occupation if you are ploughing a new furrow in an open field, difficult if you encounter stony ground and very rewarding if you get a good harvest at the end.
It was my interest in science and my experience of being brought up on a farm that led me to study veterinary medicine at Glasgow University in Scotland. After graduation, I elected to remain in the academic environment, first as a house surgeon in the surgery department at the University Veterinary Hospital, and following study for a recently introduced diploma in Veterinary Anesthesia became a veterinary anesthesiologist at the University.
When clinical trials began with this emulsion formulation I moved to the Medical Department to assist with clinical pharmacology studies and overseas clinical trials. I found it a great privilege to work with so many enthusiastic and skilled anesthesiologists in designing and managing clinical trials. This was particularly the case in the development of the first commercial device for a novel infusion system which now allows anaesthesiologists to control depth of anesthesia by targeting and adjusting a predicted blood concentration of propofol, later to be termed target-controlled infusion. Academic groups with an interest in this area welcomed the opportunity to come together to offer advice and support to get this project off the ground. I have been most impressed by the professionalism of anesthesiologists whose role in preserving vital functions in a wide range of patients, many presenting for surgery with concomitant medical conditions and a multitude of drug therapies, deserves wider recognition. In my view, this award underlines the important contribution of anesthesiologists to medicine, science and patients.
For the future, recent questions about the possible association of anaesthetic agents with postoperative delirium and cognitive dysfunction and with neonatal neurotoxicity, have stimulated research to characterise these phenomena. Emergent basic science from these enquiries, together with exploration of possible effects of anesthetic drugs on patient outcomes from cancer surgery, may in the future yield new targets for drug discovery.
