During her PhD work with the late Allan Wilson (University of California, Berkeley), King discovered that the sequences of human and chimpanzee proteins are, on average, more than 99 percent identical; DNA sequences that do not code for proteins differ only a little more. The two primates therefore are much closer cousins than suggested by fossil studies of the time. The genetic resemblance seemed to contradict obvious distinctions: Human brains outsize those of chimps; their limbs dwarf ours; and modes of communication, food gathering, and other lifestyle features diverge dramatically. King and Wilson proposed that these contrasts arise not from disparities in DNA sequences that encode proteins, but from a small number of differences in DNA sequences that turn the protein-coding genes on and off.
Just as genetic changes drive species in new directions, they also can propel cells toward malignancy. From an evolutionary perspective, the topic of breast cancer began to intrigue King. The illness runs in families and is clearly inherited, yet many affected women have no close relatives with the disease. It is especially deadly for women whose mothers succumbed to it — and risk increases for those who have a mother or sister with breast cancer, particularly if the cancer struck bilaterally or before menopause. Unlike the situation with lung cancer, no environmental exposure distinguishes sisters who get breast cancer from those who remain disease free.
By studying a rare familial cancer, Alfred Knudsen (Lasker Clinical Medical Research Award, 1998) had shown in the early 1970s how an inherited genetic defect could increase vulnerability to cancer. In the model he advanced, some families harbor a damaged version of a gene that normally encourages proper cellular behavior. Genetic mishaps occur during a person’s lifetime, and a second ‘hit’ in a cell with the first physiological liability nudges the injured cell toward malignancy. A similar story might play out in families with a high incidence of breast cancer, King reasoned. She began to hunt for the theoretical pernicious gene in 1974.
The hunt
Many geneticists doubted that susceptibility to breast cancer would map to a single gene; even if it did, finding the culprit seemed unlikely for numerous reasons. First, most cases are not familial and the disease is common — so common that inherited and non-inherited cases could occur in the same families. Furthermore, the malady might not strike all women who carry a high-risk gene, and different families might carry different high-risk genes. Prevailing views held that the ailment arises from the additive effects of multiple undefined genetic and environmental insults and from complicated interactions among them. No one had previously tacked such complexities, and an attempt to unearth a breast cancer gene seemed woefully naïve.
To test whether she could find evidence that particular genes increase the odds of getting breast cancer, King applied mathematical methods to data from more than 1500 families of women younger than 55 years old with newly diagnosed breast cancer. The analysis, published in 1988, suggested that four percent of the families carry a single gene that predisposes individuals to the illness.
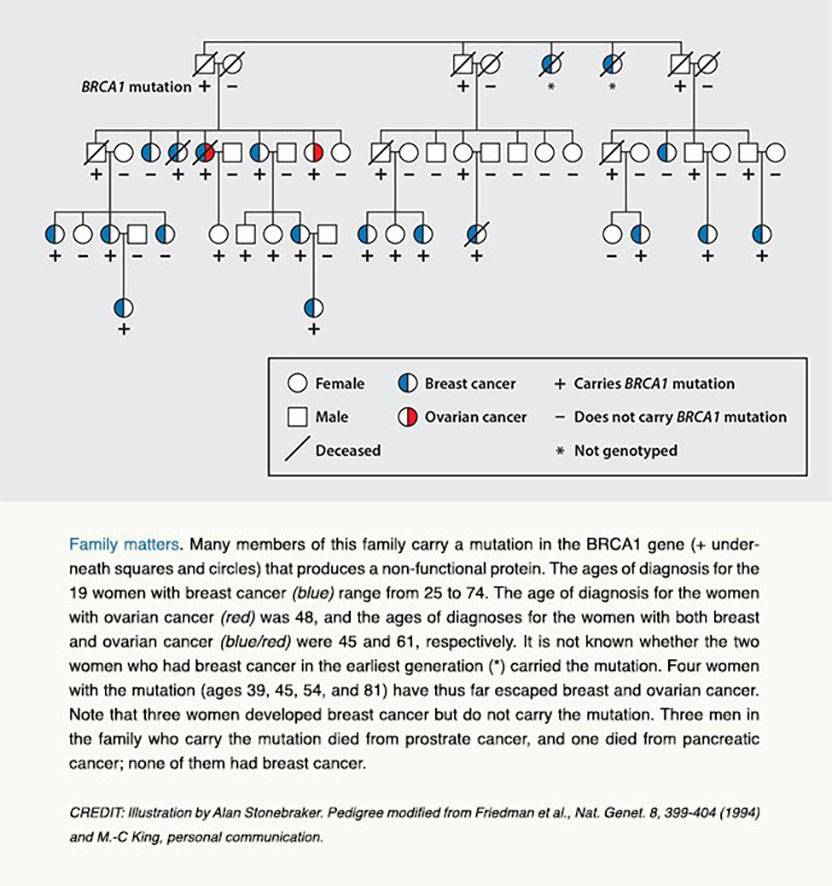
The most convincing way to validate this idea was to track down the gene. Toward this end, King analyzed DNA from 329 participating relatives with 146 cases of invasive breast cancer. In many of the 23 families to which the participants belonged, the scourge struck young women, often in both breasts, and in some families, even men.
In late 1990, King (by then a professor at the University of California, Berkeley) hit her quarry. She had zeroed in on a suspicious section of chromosome 17 that carried particular genetic markers in women with breast cancer in the most severely affected families. Somewhere in that stretch of DNA lay the gene, which she named BRCA1.
This discovery spurred an international race to find the gene. Four years later, scientists at Myriad Genetics, Inc. isolated it. Alterations in either BRCA1 or a second breast-cancer susceptibility gene, BRCA2, found by Michael Stratton and colleagues (Institute of Cancer Research, UK) increase risk of ovarian as well as breast cancer. The proteins encoded by these genes help maintain cellular health by repairing marred DNA. When the BRCA1 or BRCA2 proteins fail to perform their jobs, genetic integrity is compromised, thus setting the stage for cancer.
About 12 percent of women in the general population get breast cancer at some point in their lives. In contrast, 65 percent of women who inherit an abnormal version of BRCA1 and about 45 percent of women who inherit an abnormal version of BRCA2 develop breast cancer by the time they are 70 years old. Individuals with troublesome forms of BRCA1 and BRCA2 can now be identified, monitored, counseled, and treated appropriately.
Harmful versions of other genes also predispose women to breast cancer, ovarian cancer, or both. Several years ago, King devised a scheme to screen for all of these genetic miscreants. This strategy allows genetic testing and risk determination for breast and ovarian cancer; it is already in clinical practice.
Genetic tools, human rights
King has applied her expertise to aid people who suffer from ills perpetrated by humans as well as genes. She helped find the “lost children” of Argentina — those who had been kidnapped as infants or born while their mothers were in prison during the military regime of the late 1970s and early 1980s. Some of these youngsters had been illegally adopted, many by military families. In 1983, King began identifying individuals, first with a technique that was originally designed to match potential organ transplant donors and recipients. She then developed an approach that relies on analysis of DNA from mitochondria — a cellular component that passes specifically from mother to child, and is powerful for connecting people to their female forebears. King helped prove genetic relationships and thus facilitated the reunion of more than 100 of the children with their families.
Later, the Argentinian government asked if she could help identify dead bodies of individuals thought to have been murdered. King harnessed the same method to figure out who had been buried in mass graves. She established that teeth, whose enamel coating protects DNA in the dental pulp from degradation, offer a valuable resource when attempting to trace remains in situations where long periods have elapsed since the time of death.
This and related approaches have been used to identify soldiers who went missing in action, including the remains of an American serviceman who was buried beneath the Tomb of the Unknowns in Arlington National Cemetery for 14 years, as well as victims of natural disasters and man-made tragedies such as 9/11.
Mary-Claire King has employed her intellect, dedication, and ethical sensibilities to generate knowledge that has catalyzed profound changes in health care, and she has applied her expertise to promote justice where nefarious governments have terrorized their citizens.
by Evelyn Strauss
Key publications of Mary-Claire King
King, M.-C. and Wilson, A.C. (1975). Evolution at two levels in humans and chimpanzees. Science. 188,107-116.
Hall, J.M., Lee, M.K., Morrow, J., Newman, B., Anderson, L.A., Huey, B., and King, M.-C. (1990). Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 250,1684-1689.
King, M.-C. (1991). An application of DNA sequencing to a human rights problem. Mol. Genet. Med. 1, 117-131.
Ginther, C., Issel-Tarver, L., and King, M.-C. (1992). Identifying individuals by sequencing mitochondrial DNA from teeth. Nature Genet. 2,135-138.
Friedman, L.S., Ostermeyer, E.A., Szabo, C.I., Dowd, P., Lynch, E.D., Rowell, S.E., and King, M.-C. (1994). Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nature Genet. 8, 399-404.
King, M.-C. (2014). “The race” to clone BRCA1. Science. 343, 1462-1465.

 Pioneering scientist, social activist, humanitarian — Mary-Claire King is also foremost a free spirit who for over four decades has marched to the beat of her own drummer, animated by the impulse to solve iconic scientific puzzles, a passion for victims of disease and social injustice, and a warm humility that belies her profound impacts on science, on medicine, and on society.
Pioneering scientist, social activist, humanitarian — Mary-Claire King is also foremost a free spirit who for over four decades has marched to the beat of her own drummer, animated by the impulse to solve iconic scientific puzzles, a passion for victims of disease and social injustice, and a warm humility that belies her profound impacts on science, on medicine, and on society.

