Brian J. Druker
Oregon Health & Science University

Nicholas B. Lydon
Novartis

Charles L. Sawyers
Memorial Sloan-Kettering Cancer Center
For the development of molecularly-targeted treatments for chronic myeloid leukemia, converting a fatal cancer into a manageable chronic condition.
The 2009 Lasker~DeBakey Clinical Medical Research Award honors three scientists who developed novel treatments for chronic myeloid leukemia (CML) that converted this fatal cancer into a manageable chronic condition. By targeting the molecular underpinnings of this disease, Brian J. Druker (Oregon Health & Science University), Nicholas B. Lydon (formerly, Novartis), and Charles L. Sawyers (Memorial Sloan-Kettering Cancer Center) broke new ground in cancer therapy and radically altered the prognosis of CML patients.
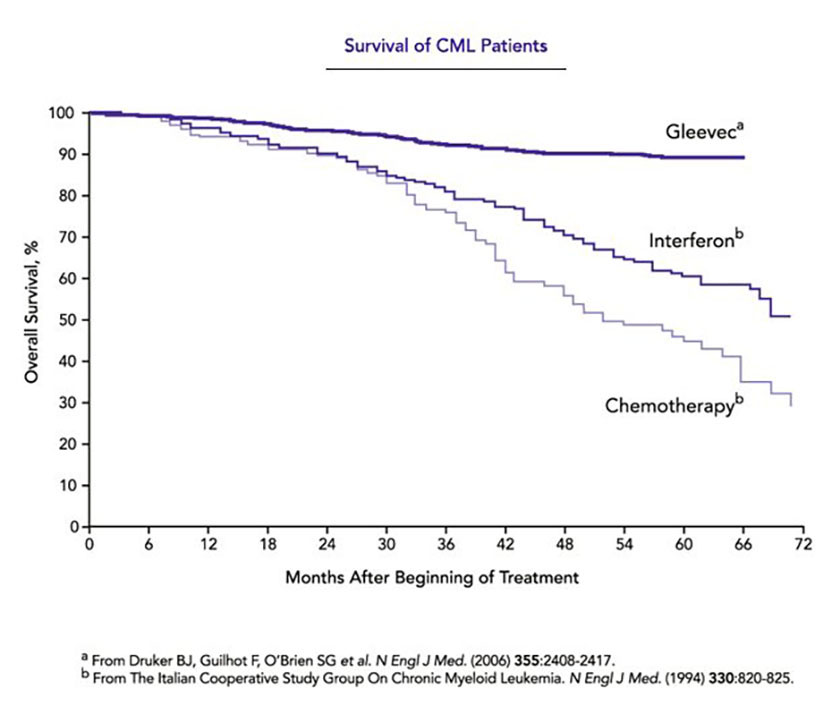
In the early phase of CML — known as the chronic stage — the body accumulates too many white blood cells, but these cells mature and function properly, and symptoms are not serious. Without treatment, the disease advances over a period of several years to a point of ‘blast crisis’, in which many immature blood and bone marrow cells accumulate — a condition that rapidly causes death. Few patients qualify for a bone marrow transplant to treat the disease, a risky prospect anyway, and, before the work of Druker, Lydon, and Sawyers, the rest were left with the drug of choice: interferon. This therapy prolongs survival by an average of only about two years and side effects are debilitating. Now, the five-year survival rate approaches 90 percent.
Award presentation by Michael Bishop
 At the turn of the 20th century, the German medical scientist Paul Ehrlich took aim at the leading killer of his time: infections. Ehrlich was aware that some diagnostic stains for bacteria failed to react with human cells. This inspired him to imagine therapeutics that would behave in the same way — “magic bullets” as he called them, aimed exclusively at infectious agents and harmless to normal human tissues. Ehrlich’s pursuit of his vision led to the first effective treatment for syphilis, but he never found a true magic bullet. He died a disillusioned man, regarding his life in science as a failure, unconsoled by the Nobel Prize that he received in 1908. The marvel of penicillin and numerous other antibiotics that embodied Ehrlich’s vision would come later.
At the turn of the 20th century, the German medical scientist Paul Ehrlich took aim at the leading killer of his time: infections. Ehrlich was aware that some diagnostic stains for bacteria failed to react with human cells. This inspired him to imagine therapeutics that would behave in the same way — “magic bullets” as he called them, aimed exclusively at infectious agents and harmless to normal human tissues. Ehrlich’s pursuit of his vision led to the first effective treatment for syphilis, but he never found a true magic bullet. He died a disillusioned man, regarding his life in science as a failure, unconsoled by the Nobel Prize that he received in 1908. The marvel of penicillin and numerous other antibiotics that embodied Ehrlich’s vision would come later.
Although rarely mentioned, Ehrlich also imagined magic bullets for cancer. But cancer is a far more subtle adversary than infections. Cancer cells are born of normal cells, a kinship that until now has caused many of our therapies to wreak havoc with the health and welfare of cancer patients. It is fair to say that ‘chemo’ has ranked among the most dreaded of all medical procedures. And as further insult, it rarely cures. Almost a century after Paul Ehrlich, magic bullets aimed at cancer were still eluding us. The prospects improved, however, with the discovery that cancer arises from the malfunction of genes. Each malfunction sets the cancer cell apart from the normal cell; each may be a keystone for the creation of a therapeutic magic bullet. The 2009 Lasker Award for Clinical Research honors three scientists who played a central role in bringing a full-blooded magic bullet for cancer to life: Brian Druker, Nicholas Lydon, and Charles Sawyers.
Acceptance remarks

Acceptance remarks, 2009 Lasker Awards Ceremony
I would like to thank the Lasker Foundation for this prestigious honor.
I began my career in cancer research nearly 25 years ago with a dream of finding better cancer treatments. I believed the future was targeted therapies where we’d kill cancer cells without harming normal cells. To do this, I believed, we needed to figure out what was triggering the growth of cancer cells and fix it. At the time, this vision was not shared by many.
But I was fortunate; I found a collaborator, Nick Lydon, who shared my vision. The project faced many hurdles, including convincing the drug company, Novartis, to go to clinical trials. I wasn’t just a researcher, isolated in my lab, I was a doctor and I had patients who desperately needed this drug to live. In 1998, we started the clinical trials with this once-a-day pill, and within six months, every single one of our patients had their blood counts return to normal. It has been over 10 years, and today patients who once had a life expectancy of three to five years are now expected to live 30 years. With Gleevec, we’ve turned a fatal cancer into a manageable disease.
One of the best rewards of my job is every week I get to see patients who are thriving because of our work. I get to hear their stories, hear about their children and grandchildren. And I am privileged to have one of my patients here from Oregon today.
I was giving a talk recently and a woman, battling colon cancer, asked me when are we going to have a Gleevec for her cancer? I gave her a typical stilted researcher answer and told her about the 30 or more years of research that went into developing Gleevec. I went on to say that I was confident that someday we would have a Gleevec for colon cancer, but that we have to be patient. Later my wife reminded me that cancer patients don’t have the luxury of patience. As always, my wife is right. If I had been patient, I would not be standing here today. And while I — like those of you here today — want to savor the success of Gleevec, I also believe that we can’t be patient.
There is urgency to the work we do. Mary Lasker understood that urgency. She was a tireless advocate for cancer research. And I am so honored to receive this award that bears her name.
We live in a time of great promise. There are incredible opportunities in cancer research. What Gleevec tells us is that by understanding cancer we can develop effective treatments. Gleevec tells us we are on the right track, but we can’t be complacent. We can’t be patient. We must seize this momentum to reach the finish line of curing cancer. Thank you for this great honor.

Acceptance remarks, 2009 Lasker Awards Ceremony
It is an enormous honor and pleasure to be here with my co-recipients to receive the Lasker~DeBakey Award.
Like many pharmaceutical discoveries, imatinib was a team effort that was built on a foundation of basic science discoveries, some of which have previously been recognized by the Lasker Foundation. I am honored to accept this award on behalf of myself and my collaborators at Ciba-Geigy, who contributed to the development of imatinib.
My interest in kinases started during my postgraduate days at the University of Dundee, where I was profoundly influenced by the work of Philip Cohen’s lab on the regulation of glycogen metabolism by reversible protein phosphorylation. Armed with a growing interest in protein kinases, I was fortunate to move into applied research at a time when pioneering studies in the oncogene field had come together to clearly implicate the Bcr-Abl kinases in the pathogenesis of CML. In retrospect, being new to the pharmaceutical industry was a big advantage, as I was naive to the complex path a drug discovery idea must take from its inception in the lab through to the clinic. Luckily, I was not alone in this journey and had the fortune of working with outstanding colleagues and mentors at Ciba-Geigy and collaborating with Brian Druker in the translation of imatinib into the clinic.
I consider myself extremely blessed in having been able to contribute to a project that has made a difference to cancer patients. I thank you for this great honor and hope that it will motivate young scientists to work on translating advances that have been made in basic research into treatments that make an impact on patients’ lives.
Acceptance remarks, 2009 Lasker Awards Ceremony
It is a humbling and unexpected honor to be here accepting the Lasker~DeBakey Clinical Research Award. Brian Druker and I met nearly 20 years ago when we were both postdoctoral fellows studying the signaling properties of the BCR-ABL enzyme. Brian was in Boston, I was in Los Angeles. I met Nick Lydon a few years later in Basel, Switzerland, when he and Brian invited me to join the effort to move the compound that would become Gleevec into the clinic. Successful partnerships are born in unpredictable ways. Who knew what would follow?
Among our trio, I am known primarily as the resistance guy. In 2001 my group discovered that patients who relapse while taking Gleevec have mutations in the BCR-ABL enzyme that prevent the drug from binding its target. This was a big surprise because all bets were on another explanation — that tumors would escape their need for BCR-ABL because they had so many other genetic mutations that could presumably drive the growth of the tumor. Instead, the answer was elegantly simple. The tumor does everything in its power to maintain BCR-ABL activity. In hindsight, this was clear evidence of a general principle we now call oncogene addiction that guides much of cancer drug development today.
Many people played a role in the remarkable convergence of events that allowed us to move so quickly from discovering the cause of resistance in 2001 to a solution to overcome it just a few years later. Pasteur said that “chance favors the prepared mind.” I credit Owen Witte, my postdoctoral mentor, for ‘preparing’ my mind to connect the various pieces of the resistance puzzle that we faced first in the clinic, then in the laboratory. Mercedes Gorre and Neil Shah, who are both here today, conducted the experiments in my lab that led to the discovery of the resistance mutations and courageously persevered when their initial results were challenged. John Kuriyan solved the structure of Gleevec bound to ABL, then partnered with us to understand how the many different mutations we were finding in patients confer resistance. Only through that collaboration did we come to the hypothesis that an inhibitor that binds BCR-ABL differently might offer a common solution against the many different Gleevec-resistant mutations. In an amazing coincidence, Frank Lee and Rob Kramer at Bristol Myers Squibb had a compound with precisely the properties we were imagining but that they had originally discovered for a different purpose. Four years later that compound, dasatinib or Sprycel, was approved by the FDA for Gleevec-resistant chronic myeloid leukemia.
In closing, I thank the patients who participated in these clinical trials, many of whom have sent me messages of joy ever since the Lasker announcement. I also thank my parents, my brother and sister, and my wife and children, all here today, for supporting my dedication to this work.
Interview with Brian J. Druker, Nicholas B. Lydon, and Charles L. Sawyers
Video Credit: Susan Hadary