
Akira Endo
Biopharm Research Laboratories, Inc.
For the discovery of the statins — drugs with remarkable LDL-cholesterol-lowering properties that have revolutionized the prevention and treatment of coronary heart disease.
The 2008 Lasker~DeBakey Award for Clinical Medical Research honors a scientist who discovered statins — drugs with remarkable LDL-cholesterol-lowering properties that have revolutionized the prevention and treatment of coronary heart disease (CHD). Akira Endo (Biopharm Research Laboratories, Inc., Tokyo) sifted through thousands of organisms, hunting for natural substances that block a key enzyme in the biochemical pathway that produces cholesterol, a major contributor to CHD. Remarkably, the compound that Endo found lowers concentrations of LDL (the bad cholesterol) but not HDL (the good cholesterol) in the bloodstream of animals and humans. His work stimulated Merck, Sharp & Dohme Research Laboratories (Merck) to launch a drug-development program that led, 20 years ago, to the first statin approved for medical use. This advance paved a path for other pharmaceutical companies to follow.
LDL in the bloodstream can form fatty deposits that narrow blood vessels. When this process occurs in arteries that deliver blood to the heart, it can lead to CHD, the major cause of chest pain and heart attack and the top killer in the industrialized world. In the US, more than 450,000 people died from CHD in 2004. In 2005, the American Heart Association estimated that 16 million Americans had CHD and 1.2 million would have a new or recurrent heart attack that year. Statin use is increasing — 30 million people worldwide are taking them — and has begun to make a dent in those numbers. The drugs dramatically reduce the risk of CHD and its associated life-threatening events. Furthermore, studies with statins have erased long-standing doubt about the possibility of safely reducing cholesterol quantities with pharmaceutical agents.
Promising early results
In the 1950s and 1960s, epidemiological studies suggested a link between LDL cholesterol in the blood and CHD. Reducing LDL would shrink the incidence of this illness, some experts reasoned. However, many scientists and clinicians questioned whether doing so would stir trouble. Cholesterol is a crucial component of the membranes that encase our cells, and it serves as a raw material for other essential molecules, including some hormones and the sheath that insulates nerves. Further exacerbating uncertainty, most early agents that reduced cholesterol quantities in the blood cut concentrations only modestly and triggered unwelcome side effects. Therefore, skepticism simmered about the wisdom of lowering cholesterol concentrations.
Despite the concerns, efforts pushed forward. Humans acquire cholesterol from food and we also make it in our bodies, mostly in the liver. Dietary interventions to limit cholesterol intake had met with poor success and the idea of short-circuiting our ability to produce the compound seemed attractive. An enzyme called HMG-CoA reductase plays a central role in the manufacture of cholesterol. In the biochemical pathway that generates cholesterol, it converts a precursor molecule, HMG-CoA, into the next compound, mevalonate.
By 1971, when Endo began his work (at Sankyo Company in Tokyo), thwarting the reductase seemed like a possible way to keep the body’s cholesterol production in check. Endo had a novel idea about how to find substances that block the reductase. Aware that organisms can secrete compounds with powerful biological activities — presumably to kill their competitors — Endo proposed that some creatures might spit out chemicals that foil the reductase and thus impede a vital activity of their neighbors.
Over the next two years, Endo and colleagues grew more than 6000 fungi, harvested the broth in which each had grown, and tested whether the material could interfere with an early step of cholesterol synthesis in a test tube. They then separated its components from one another, keeping track of the active material. By this and additional methods, he purified a substance from the fungus Penicillium citrinum, called mevastatin or compactin, that blocks the reductase.
Additional analysis revealed that compactin resembles HMG-CoA and competes strongly with it for the site on the enzyme that catalyzes HMG-CoA’s conversion to mevalonate. This capacity to bind specifically to the spot normally reserved for the enzyme’s substrate suggested that the potential drug would not randomly clasp molecules in cells and disrupt other important activities.
Endo wondered whether compactin would reduce enzyme activity in animals, as it does in test tubes. In 1979, he showed that the compound dramatically lowers blood cholesterol in dogs and monkeys with no obvious toxic effects.
While these results were coming in, Endo and physician Akira Yamamoto (National Cardiovascular Center in Osaka) gave compactin to patients with extremely high LDL-cholesterol levels. In 1980, they reported that it cut LDL in the blood by an average of 27%. The next year, Hiroshi Mabuchi of Kanazawa School of Medicine published similarly promising findings. Furthermore, he established that the compound did not perturb quantities of a molecule that is involved in the cell’s energy-production system whose concentrations experts thought might drop when HMG-CoA activity was stymied. These observations fed hope that compactin could reduce cholesterol without causing harm.
At the same time, different researchers were demonstrating the mechanism by which the drug acted, which provided a rationale for how it could operate safely. In 1974, Michael Brown and Joseph Goldstein (Lasker Basic Medical Research Award and Nobel Prize, 1985) at the University of Texas Southwestern Medical School discovered that cell-surface molecules known as LDL receptors on liver cells grab LDL in the blood. In 1981, they showed that, when statins lower reductase activity and cholesterol production wanes, cells place additional LDL receptors on their surfaces. Statins thus harness a normal mechanism by which cells drain cholesterol from the blood, yet ensure a steady supply of the molecule internally for vital activities.
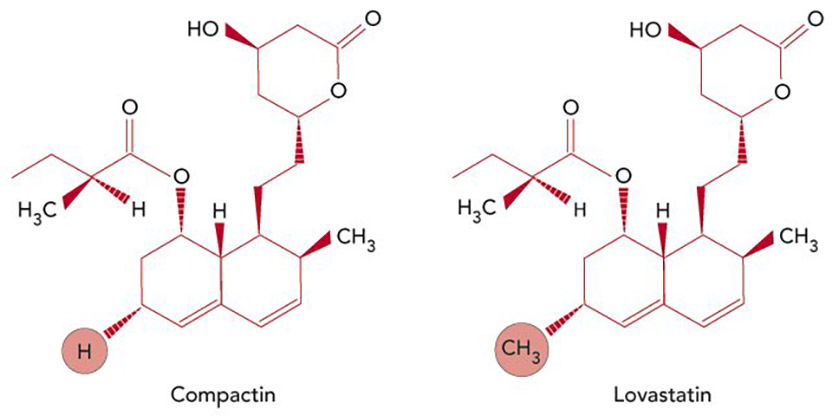
Reducing cholesterol. Compactin, the first statin, discovered by Akira Endo (left); Lovastatin, the first statin approved for clinical use, developed by Merck (right). The compounds differ by a single methyl group at the circled position. [Credit: Carin Cain]
Statins in the clinic
In 1978, Endo left Sankyo and joined the faculty of Tokyo University of Agriculture and Technology. There, he isolated additional HMG-CoA reductase inhibitors, including one called monacolin K, from another fungus. Merck scientists, led by Alfred W. Alberts, independently identified — from a different organism — the same compound, which they called mevinolin (and subsequently renamed lovastatin). The company had been pursuing statin-related research since soon after Endo published his first papers in 1976. At that time, P. Roy Vagelos, then President of Merck Research Laboratories, had signed an agreement with Sankyo and obtained samples of compactin from Endo, with which Merck confirmed Endo’s results and launched its own program.
In April 1980, Merck began clinical studies of lovastatin. Several months later, rumors emerged from Sankyo that its drug (compactin) caused tumors in dogs. Because of this uncertainty, Merck halted the studies of its drug (lovastatin). However, experts in the field urged Merck forward. The dogs at Sankyo were receiving exceptionally high doses of compactin and Sankyo provided no data for others to assess. Japanese investigators were witnessing dramatic benefits with compactin, particularly to individuals who have a genetic alteration in one of their two copies of the gene for the LDL receptor and thus carry colossal quantities of circulating cholesterol; these patients are extremely difficult to treat.
Several years later, Merck picked up its clinical trials on lovastatin. In 1987, the FDA approved the drug (Mevacor®).
The success of compactin and lovastatin inspired efforts to improve the statins by chemically modifying the natural compounds or crafting synthetic ones. Because the statins curb LDL so much more than any of the existing treatments, it became possible to demonstrate unequivocally that the drugs decrease CHD events without serious side effects. For example, in 1994, the Scandinavian Simvastatin Survival Study (4S study) showed that treatment with simvastatin (the second Merck statin) — for as short a period as 5 years — lowered LDL levels by 35%; this reduction was associated with a drop of 40% in CHD deaths. A 2005 analysis of more than 90,000 people in multiple 5-year randomized studies obtained similar results. Furthermore, this work established that people reap these benefits without an increase in cancer, other diseases, or deaths from any cause.
Statins are now routinely used to prevent and treat CHD throughout the world. Although CHD is aggravated by multiple risk factors in addition to high LDL — cigarette smoking, high blood pressure, obesity, and diabetes — reducing LDL levels alone makes a significant impact.
By discovering statins, Endo ushered in a new era in preventing and treating CHD, the leading cause of death in the US and a major source of human suffering. His work has touched millions of people and promises to prolong and improve the lives of many millions more.
by Evelyn Strauss
Key publications of Akira Endo
Endo, A., Kuroda, M., and Tanzawa, K. (1976). Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolmic activity. FEBS Lett. 72, 323-326.
Endo, A., Kuroda, M., and Tsujita, Y. (1976). ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J. Antibiot. (Tokyo). 29, 1346-1348.
Endo, A., Tsujita, Y., Kuroda, M., and Tanzawa, K. (1977). Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Eur. J. Biochem. 77, 31-36.
Tsujita, Y., Kuroda, M., Tanzawa, K., Kitano, N., and Endo, A. (1979). Hypolipidemic effects in dogs of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Atherosclerosis. 32, 307-313.
Yamamoto, A., Sudo, H., and Endo, A. (1980). Therapeutic effects of ML-236B in primary hypercholesteromia. Atherosclerosis. 35, 259-266.
Endo, A. (1992). The discovery and development of HMG-CoA reductase inhibitors. J. Lipid Res. 33, 1569-1582.
Award presentation by Joseph Goldstein
 The human body produces tens of thousands of different molecules. Several of them — DNA, hemoglobin, insulin, estrogen — are household names. But none has captured the public’s imagination like cholesterol. Cholesterol is the quintessential celebrity molecule: it appears regularly on the front pages of USA Today and the New York Times, on the covers of Time and Newsweek, and it’s the subject of endless Jay Leno jokes, New Yorker cartoons, and cocktail party conversations. Many of you in the room know your cholesterol level but can’t remember your wedding anniversary.
The human body produces tens of thousands of different molecules. Several of them — DNA, hemoglobin, insulin, estrogen — are household names. But none has captured the public’s imagination like cholesterol. Cholesterol is the quintessential celebrity molecule: it appears regularly on the front pages of USA Today and the New York Times, on the covers of Time and Newsweek, and it’s the subject of endless Jay Leno jokes, New Yorker cartoons, and cocktail party conversations. Many of you in the room know your cholesterol level but can’t remember your wedding anniversary.
Like Madonna, cholesterol has a good side and a bad side. It’s a Dr. Jekyll and Mr. Hyde-type molecule; it’s vital and lethal at the same time. Cholesterol’s vital role is its essential function in maintaining the integrity of the membranes that surrounds every cell in our body. Cholesterol becomes lethal when it accumulates in the blood in the form of a lipoprotein particle called LDL. LDL is the toxic particle that initiates the atherosclerotic process in arteries, which leads after many years to coronary heart disease and heart attacks.
Coronary disease is responsible for more than one-third of all deaths in the developed world. It is the number one killer in the United States. As many as 16 million Americans alive today have coronary disease, and 1.2 million will have a new or recurrent heart attack this year. An overwhelming body of evidence — genetic, experimental, epidemiological, and therapeutic — has established a causal link between the cholesterol-carrying LDL particle and coronary disease. Building on that knowledge, scientists and the pharmaceutical industry have successfully developed a remarkably effective class of drugs — the statins — that lower LDL-cholesterol levels in blood and reduce the frequency of heart attacks.
This year’s Lasker~DeBakey Clinical Award is given to the scientist — Akira Endo — who discovered the first statin and demonstrated its clinical efficacy. As a child growing up on a farm in Northern Japan, Endo became fascinated with mushrooms and other molds, and as a young boy he read several biographies of Alexander Fleming and the discovery of penicillin in a mold. After obtaining his PhD in biochemistry in 1957, Endo joined the Sankyo Co. in Tokyo as a research scientist. His project was to identify enzymes produced by molds that make fruit juices less pulpy. After 12 years on this project, Endo’s efforts led to several commercially successful products, and the Sankyo Co. rewarded him with two bonuses.
The first bonus was a two-year sabbatical in the United States, where he worked on phospholipids in the department of a great biochemist, Bernard Horeker at the Albert Einstein Medical Center here in New York City. Sankyo’s second bonus to Endo — one that is rarely if ever granted to a scientist in a pharmaceutical company — was the opportunity to work for two years on a project totally of his own choosing. So, in 1970, Endo and his colleague, Masao Kuroda, began a search for inhibitors of cholesterol synthesis, reasoning that a decrease in cholesterol production in the body would lower cholesterol levels in the blood and thus decrease coronary disease.
Inspired by Fleming’s success with molds, Endo began searching fungal cultures for secreted natural products that would inhibit cholesterol synthesis in an homogenate of rat liver. Endo’s assay was anything but simple: it was complicated, cumbersome, and capricious, involving the conversion of a radioactive two-carbon precursor molecule into the final 27-carbon cholesterol, a sequence of reactions requiring the concerted performance of 25 different liver enzymes. For two years, Endo and Kuroda toiled day and night, screening through 6000 different fungal extracts, but with no success. Finally, as their deadline was approaching and the project was winding down, on March 15, 1972, Endo hit pay dirt — literally and figuratively — in a strain of Penicillium citrinum that grew in the rice fields near Kyoto. The extract from this mold produced a powerful inhibitory activity. Historians of science will come to view the Ides of March, 1972, as the day that cholesterol was assassinated. Atherosclerosis, beware!
Even though the Sankyo Co. in 1972 was not keenly interested in developing cholesterol-lowering drugs, they nonetheless permitted the project to continue. Endo spent the next several years purifying the inhibitor, working out its structure, and identifying HMG CoA reductase as the target enzyme in the cholesterol pathway that was inhibited by their drug, known today as compactin. In 1976, Endo published two papers reporting the discovery and characterization of compactin, the first statin.
The next chapter in the statin story involved a series of animal experiments in which Endo showed that compactin lowered the plasma cholesterol level in dogs and in monkeys. The success of these studies led Endo to a surreptitious clinical study in patients — a study that was neither sanctified nor sanctioned by Sankyo.
Not being a clinician, Endo procured the collaboration of Akira Yamamoto, an astute physician at the National Cardiovascular Centre in Osaka. Yamamoto gave oral compactin to 11 patients with genetic forms of hypercholesterolemia, all of whom had been poorly responsive to available cholesterol-lowering drugs. A dramatic result was observed: compactin was extremely effective in lowering plasma cholesterol. Yamamoto had never seen such an effective response. Endo and Yamamoto published their pioneering studies in an obscure journal in 1980, one year after Endo had left Sankyo for an academic position at Tokyo Noko University. One year later, in 1981, the second clinical study on compactin was published in a not-so-obscure journal, the New England Journal of Medicine, and the world took note. A Japanese team, led by Hiroshi Mabuchi of Kanazawa University, reported that compactin reduced LDL, the bad cholesterol, but did not lower HDL, the good cholesterol. I’ll come back to the significance of this key observation in a moment.
By early 1978, many pharmaceutical companies, although originally skeptical about the safety of inhibiting cholesterol synthesis in the body, had learned of Endo’s results and jumped on the statin bandwagon, feverishly searching microorganisms for HMG CoA reductase inhibitors. The race to the finish line was won by the Merck Sharp & Dohme Research Laboratories. Led by biochemist Alfred W. Alberts, Merck scientists in 1979 identified a molecule, secreted by an Aspergillus mold, that differed from compactin by only four atoms. Merck’s molecule, called lovastatin (Mevacor®), became the first statin to be approved for human use eight years later in 1987. Today, more than a half a dozen statins (both natural products and totally synthetic versions) have been developed and commercialized, the most popular being Pfizer’s atorvastatin (Lipitor®) and Merck’s simvastatin (Zocor®).
Statins have now been tested in 14 large randomized multicenter trials, involving an unprecedented 90,000 middle-aged adults who were followed for five years. The results in all 14 studies have been astonishingly consistent: treatment with statins lowers plasma LDL levels by 25-35% and reduces the frequency of heart attacks by 25-30%. The percentage reduction in coronary events would be even more dramatic if the treatment were longer — say 10 or 15 years — and if statin therapy were started 20 years earlier — say age 30 or 40 — when clinically silent atherosclerotic plaques are fewer and smaller.
A noteworthy aspect of the 14 statin trials is that no major harmful effects of lowering cholesterol were observed in any of the studies. The remarkable safety of statins derives from their unique mechanism of action. When a statin is ingested, the drug is absorbed by the intestine and routed primarily to the liver where it binds and inhibits HMG CoA reductase, lowering cholesterol production. This decrease in liver cholesterol triggers a compensatory feedback loop that increases the number of LDL receptors displayed on the liver cell membrane. These LDL receptors grab onto LDL, remove it from the blood, and deliver it to the interior of the cell, where the LDL is digested and its released cholesterol becomes available for metabolic purposes. The net effect is that the amount of cholesterol in the liver is maintained at a normal level while at the same time the level of LDL-cholesterol in blood is kept low. As an added dividend, the elevated LDL receptors don’t grab HDL, and the blood level of the good cholesterol doesn’t drop. If all drugs worked in such a perfect way, the drug industry would be in perpetual pharmaceutical heaven.
The statins are the largest-selling class of drugs currently taken by patients throughout the world. Sales for this one class of drugs in 2007 were $34 billion — $34 billion. That’s a hell of a lot of money — even by Mayor Bloomberg’s standards. Today, an estimated 30 million people worldwide are taking statins, including 30% of all Medicare patients in the United States. The millions of people whose lives will be extended through statin therapy owe their good fortune to the immense contributions of Akira Endo. Without the exuberant unpredictability of Endo’s hunt through 6000 fungal extracts 38 years ago, statins might never have been discovered. Moreover, statins might never have become approved drugs were it not for the pharmaceutical clout of Merck, which in those days was under the leadership of Roy Vagelos and his talented team of colleagues that included, in addition to Al Alberts, Jonathan Tobert, Richard Monaghan, George Albers-Schonberg, James MacDonald, and Edward Scolnick.
Despite the triumphant success of Endo’s approach to drug discovery, the major pharmaceutical companies have largely abandoned the screening of natural products created by evolution in favor of the screening of synthetic chemical libraries created by modern-day chemical biologists. No random man-made library, no matter how large, would ever be expected to yield an HMG CoA reductase inhibitor with the potency and selectivity of Endo’s first statin. The complex structure of compactin, containing seven asymmetric carbon atoms, evolved over billions of years of evolution to target HMG CoA reductase’s catalytic site by mimicking its natural substrate (HMG CoA). To paraphrase the second law of Leslie Orgel, the great British chemist, evolution is smarter than any chemical biologists.
So now for the $34 billion question. Does Dr. Endo take a statin? In an interview with the Wall Street Journal three years ago, Endo told the reporter that his LDL-cholesterol was elevated at 155 (a normal level being under 100), but he was not taking a statin at that time. In offering an explanation for why the discoverer of statins would dismiss his own discovery, Endo invoked a Japanese proverb: “The indigo dyer wears white trousers.” But, as you’ll see in a moment, Dr. Endo is now wearing black trousers. I’ll leave it to him to tell us whether he’s now taking a statin and which one.
Acceptance remarks by Akira Endo

Acceptance remarks, 2008 Lasker Awards Ceremony
It is a great honor for me to receive the 2008 Lasker Award in Clinical Medical Research. I thank the members of the jury and the foundation that made it possible.
Four decades ago, from 1966 to 1968, I studied at the Albert Einstein College of Medicine here in New York. Even before that time I was interested in cholesterol metabolism, and during my stay here, I learnt much about cholesterol both in my daily work and life.
My experience of living in New York made me realize the importance of developing an effective cholesterol-lowering agent as a research area and stimulated my interest in this field. As is now known, cholesterol-lowering drugs are crucial in the prevention of coronary heart disease.
If I had not stayed and studied in New York four decades ago, I would never have discovered statins. Thanks to my stay and study in New York, I was able to make a contribution toward helping people. Thank you very much.
Interview with Akira Endo
Video Credit: Susan Hadary
