
Ralph M. Steinman
The Rockefeller University
For the discovery of dendritic cells — the preeminent component of the immune system that initiates and regulates the body’s response to foreign antigens.
The 2007 Albert Lasker Award for Basic Medical Research honors the scientist who discovered dendritic cells, the preeminent component of the immune system that initiates and regulates the body’s response to foreign antigens. At a time when conventional wisdom pointed in other directions, Ralph Steinman proposed that these cells propel other immune cells into action and then pursued that possibility with careful and dedicated experimentation. He revolutionized our understanding of the events that instigate an immune response and unlocked the entire field of T-cell activation.
In the last 34 years, Steinman’s work has revealed that dendritic cells scour the environment for microbial invaders and stimulate the T cells of the immune system to respond. These T cells, in turn, prompt other immune cells to eliminate the threat. Steinman devised techniques that generate large numbers of dendritic cells, which fueled a research boom in this area. His work spawned a major discipline, with hundreds of investigators worldwide studying these cells and their roles in multiple aspects of immune regulation.
Branching out in the immune system
Since the dawn of immunology, at the end of the 19th century, scientists have grappled with the question of how vertebrates respond to the tremendous array of pathogens that they encounter. In the 1950s, immunologists proposed that immune cells use receptor molecules on their external surfaces to recognize invading germs and other foreign particles, or antigens. Each receptor differs slightly from the next and can ‘see’ a different antigen. Vast numbers of immune cells exist in the body prior to any infection; together, they detect all antigens that an organism meets. Once an antigen contacts its receptor, the associated immune cell multiplies to create an army that rises up to attack that specific antigen.
By 1970, when Steinman began his work, this ‘clonal selection’ theory was well accepted. Scientists had discovered that lymphocytes, a group of cells in the blood and other immune-related tissues, play key roles in fighting microbial trespassers. For example, B cells produce antibodies, which attach to bacteria and mark them for destruction by other components of the immune system. T cells perform numerous tasks: Some types kill virus- and bacteria-infected cells, whereas others incite B cells to manufacture antibody.
To better understand lymphocytes’ functions and interactions, researchers wanted to re-create aspects of the immune response in culture dishes. When they attempted to do so, they encountered a mystery: Adding antigens to lymphocytes in culture dishes did not trigger the cells’ duplication. Apparently, something in the body that stimulated lymphocytes to respond to antigen was absent from the culture dish. Steinman set out to find the missing piece.
This issue lay at the center of immunological study at the time. Scientists speculated that lymphocytes could detect foreign agents only if an unidentified ‘accessory’ cell displayed the microbe’s antigens on its surface. Macrophages, which engulf and digest cellular debris and pathogens, seemed like strong contenders for this job. B cells and T cells also were candidates. However, convincing evidence remained elusive. For example, if macrophages could activate T cells, the more macrophages a sample contained, the more powerful should be its stimulating power; but the abundance of macrophages did not correlate with T-cell-activating capacity.
Working with the late Zanvil Cohn at the Rockefeller University, Steinman harvested a cellular mixture from mouse spleen that was known to spur T cells to divide in culture dishes. When he peered through the microscope at his spleen-derived substance, he noticed not only macrophages and other established immune cells, but rare, irregularly shaped cells that no one had described before. They moved in a distinctive way: Long projections emerged and floated around before retracting, giving the cells a dynamic star-like appearance. This branching behavior inspired Steinman to dub them dendritic cells, derived from the Greek word for tree. The cells differed from other immune cells in structure and behavior. For example, their surfaces didn’t carry molecules that typify macrophages, and they poorly internalized material from their environment. Unlike macrophages, they detached from plastic and glass culture dishes after growing overnight in the lab. Steinman proposed that this newly described cell performs a distinct physiological chore.
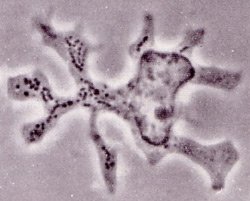
Ready for action. A dendritic cell initiates the immune response by using its long protrusions to present antigen to passing T cells. [Reproduced from The Journal of Experimental Medicine 1973;137:1142-1162. Copyright 1973 The Rockefeller University Press.]
A shot in the arm for T cells
By 1978, Steinman had exploited the properties that distinguish T cells, B cells, macrophages, and dendritic cells from one another to separate the spleen mixture into its components. Then he — with his trainees, who included Margaret Witmer, Michel Nussenzweig, Wesley van Voorhis, and Kayo Inaba — added back each cell type individually to lymphocytes in culture dishes.
Tiny quantities of dendritic cells incited T cells to reproduce and kill host cells that bore foreign antigen. Dendritic cells’ stimulatory power was more than 100-fold greater than that of B cells, T cells, or macrophages. As few as 0.5 dendritic cells per 100 T cells generated maximum proliferation. No one had anticipated that any cell could so efficiently goad T cells into action.
In 1985, Steinman tested whether this property depended on a long-term association between T cells and dendritic cells. He mixed T cells with antigen and dendritic cells. Then he isolated the T cells and added them to B cells, which multiplied and produced antibody in response. This experiment showed that the dendritic cells stably altered the T cells, triggering them to mature in such a way that they could stimulate B cells, even after the dendritic cells had disappeared.
The work thus far indicated that dendritic cells could activate T cells in culture dishes. But Steinman wanted to make sure that he was studying a phenomenon — priming of the immune system — that occurred in an intact animal’s body. He exposed dendritic cells to antigen in culture; then he washed away free antigen and injected the dendritic cells into mice. The animals mounted a strong immune response, converting naïve T cells into ones that reacted strongly to antigen. This observation and others showed that dendritic cells with pre-loaded antigen were sufficient to elicit an immune reaction in animals. Analysis of cell-surface molecules revealed that the T cells produced by the mice were stimulated by the injected cells rather than by dendritic cells that had previously resided in the animals. Thus, researchers could sensitize T cells of an animal to an antigen of choice by inoculating with dendritic cells that had been exposed to that antigen; Steinman pointed out that this prospect holds extraordinary therapeutic implications, an idea that investigators are pursuing today.
The two faces of dendritic cells
These observations and others also highlighted a feature of dendritic cells that conflicted with an originally described characteristic — the inability to take up particles from their surroundings. To present antigens to T cells, dendritic cells had to overcome this apparent limitation, a property that initially distinguished them from macrophages. In a separate line of studies, Steinman had begun to solve this conundrum. He discovered that so-called Langerhans cells, which had been identified in 1868, served as dendritic-cell precursors. This and other work led him to propose a scenario for dendritic-cell specialization in the 1990s. After capturing antigen, immature cells from the skin and elsewhere begin to display it on their surfaces. In the presence of stimulatory factors from the environment and other immune cells, they develop into dendritic cells. During this process, dendritic cells lose the capacity to ingest foreign agents. The dendritic cells that sensitize T cells — and the ones Steinman originally characterized — can no longer slurp antigen.
Maturing dendritic cells migrate from tissues such as skin to the lymph nodes, where the body ratchets up immune activities in response to specific invaders. In the mid 1980s, Steinman had noticed dendritic cells at the exact sites in the lymph nodes where T cells percolate through, awaiting instructions about whether their services are needed; dendritic cells thus reside in the ideal location for initiating immunity. A dendritic cell’s long extensions constantly probe the parade of T cells until they contact their target: a T cell whose antigen ‘matches’ that on the dendritic cell’s surface.
Tumors, trespassers, and tolerance
By the end of the 1980s, dozens of labs were studying dendritic cells — but investigations were hampered by scant supplies. Only about 1 percent of mouse spleen cells are dendritic cells. In the early 1990s, Steinman’s group and several others devised ways to prepare large amounts of dendritic cells. This breakthrough made the cells widely accessible, and the field exploded. Scientists have expanded their studies along multiple avenues and are now delving into potential therapeutic uses of dendritic cells.
The capacity to load antigens onto dendritic cells that then prime T cells in an animal raised the possibility of using dendritic cells to fight cancer. In one version of this scheme, tumor cells are removed from an individual and delivered to dendritic cells from the same person in culture dishes. The dendritic cells are then injected back into the patient’s body. This procedure should boost the immune system with cells whose primary mission is to attack that particular person’s tumor. Preliminary results are promising: The method shrinks tumors in experimental animals. The approach holds strong appeal because the dendritic cells strike multiple tumor components simultaneously; in contrast, drugs tend to focus on one cancer-related pathway at a time. Dozens of clinical studies are under way and the area is ripe for development, due in large part to Steinman’s experimental work and advocacy.
Scientists are exploring similar strategies to create dendritic cell-based vaccines against pathogens. Initial observations suggest that such a tactic might thwart HIV infections. This line of investigation could prove especially fruitful, as HIV is particularly insidious with regard to dendritic cells. Steinman demonstrated that dendritic cells provide a safe haven for replicating HIV-1 and can transmit the virus to T cells. Thus, in the natural setting, dendritic cells help spread HIV-1 rather than quash it.
The importance of dendritic cells extends beyond their capacity to initiate an immune response. They help induce tolerance, the process by which animals learn to ignore their own cells. Scientists might therefore adapt dendritic cells for clinical use in autoimmunity, allergy, and transplantation medicine. These dual roles — of immune stimulation and silencing — bolster dendritic cells’ standing as a central modulator of the immune response.
The conceptual framework and practical methodologies that Steinman pioneered not only cracked open the early steps of immune activation, but unveiled mechanisms by which our bodies tune their assaults against particular microbes. Scientists now know that dendritic cells adjust the immune reaction by rousing different classes of T cells, depending on the specific signaling molecules that are carried by other cells and the environment. Steinman’s work launched an entire field. He defined the basic biology of dendritic cells and formulated the therapeutic applications of his discoveries.
by Evelyn Strauss
Key publications of Ralph Steinman
Steinman, R.M. and Cohn, Z.A. (1973). Identification of a novel cell type in peripheral lymphoid organs of mice. J. Exp. Med. 137, 1142–1162.
Nussenzweig, M.C., Steinman, R.M., Gutchinov, B., and Cohn, Z.A. (1980). Dendritic cells are accessory cells for the development of anti-trinotrophenyl cytotoxic T lymphocytes. J. Exp. Med. 152, 1070–1084.
Inaba, K. and Steinman, R.M. (1985). Protein-specific helper T-cell formation initiated by dendritic cells. Science. 229, 475–479.
Schuler, G. and Steinman, R.M. (1985). Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J. Exp. Med. 161, 526–546.
Inaba, K., Metlay, J.P., Crowley, M.T., and Steinman, R.M. (1990). Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 172, 631–640.
Pope, M., Betjes, M.G.H., Romani, Hirdman, H., Cameron, P.U., Hoffman, L., Gezelter, S., Schuler, G., and Steinman, R.M. (1994). Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 78, 389–398.
Banchereau, J. and Steinman, R.M. (1998). Dendritic cells and the control of immunity. Nature. 392, 245–252.
Steinman, R.M. and Banchereau, J. (2007). Taking dendritic cells into medicine. Nature. In Press.
Award presentation by Joseph Goldstein

Award presentation by Joseph Goldstein
We live in a dangerous world, surrounded and threatened by two types of foreign invaders — one political and the other microbial. Protection against these invaders depends on the efficient operation of two different systems of defense — one dedicated to our nation’s security and the other to our body’s immunity. As I discuss the work of this year’s Lasker Basic Award winner, you’ll see that the immune system shares many features with the government’s security system, albeit with notable differences in their effectiveness.
Our national security is organized around a two-tier operation — the CIA and the FBI. In a perfect world, these two agencies would interact and cooperate. The CIA’s mission would be to identify potential enemies, gather information on them, and analyze the intelligence for presentation to the second tier, the FBI. The FBI would receive the CIA’s intelligence, act on it, and eliminate the foreign invaders before any damage is done. But, unfortunately, we don’t live in the fictional world of movies and novels, and the CIA and FBI more often than not fail to connect the dots.
The man whom President Truman appointed as the first CIA director was a fellow Missourian whose main claim to fame was that he had run Piggly Wiggly supermarkets. In a White House ceremony, Truman presented his new CIA chief with a black cloak and a wooden dagger. J. Edgar Hoover, the FBI chief at the time, was not amused.
President Eisenhower’s view of the CIA was less jocular than Truman’s. When Eisenhower left office in 1961, he reprimanded the fifth CIA director, Allen Dulles, telling him: “I will leave my successor a legacy of ashes.” To this day, our nation’s security system remains in shattered disarray — true to Eisenhower’s prophesy.
Now to our body’s immune system, which like our nation’s security system, is a two-tier operation that gathers intelligence and then eliminates the enemy. But there is one difference between the two systems: Our immune system was created — not by a US president, but by Mother Nature — and it has been perfected, not by 60 years of congressional hearings but by 500 million years of evolution. Evolution has left us a “legacy of marvels” — a marvelous molecular machine that protects us against the millions of microbial invaders that assault the human body every day. To quote the second law of Leslie Orgel, the great British chemist, “evolution is smarter than you are.”
This year’s Basic Award winner, Ralph Steinman, is a scientific sleuth, who without cloak and dagger uncovered the intelligence-gathering component of our immune system — a monumental achievement in the 200-year history of immunology.
To explain Steinman’s discovery, I first have to define the term antigen, which is any foreign or abnormal substance, like a bacteria, a virus, or a tumor cell, that stimulates the body to produce antibodies. Antigens are detected by a network of so-called antigen-presenting cells, which constitute the intelligence-gathering component of the immune system. Antigen-presenting cells are stationed like sentinels beneath all the body’s exposed surfaces — the skin, airways, gastrointestinal tract, and genital tract. When a pathogen breaks through one of these epithelial barriers and invades the body, the antigen-presenting cells ingest the invader and fragment its antigenic proteins into small peptides, which are then displayed on their cell surface like a billboard. The antigen-presenting cells now become activated and undergo a vigorous maturation: They become uprooted from their local environment and travel to the nearest lymph node. Here, each dendritic cell presents its antigen to a resting lymphocyte called a virgin T cell, which responds by proliferating at a rapid rate, secreting cytokines, and differentiating into one of two types of mature T cells — a helper T cell or a killer T cell. The job of helper T cells is to instruct B cells to manufacture antibodies for neutralizing pathogens throughout the body. The job of killer T cells is to destroy body cells infected with pathogens. I’ll come back in a moment to how the antigen-presenting cell conveys the proper instructions to the virgin T cell, telling it whether the body needs antibodies or cell killing.
When Steinman began his research in the early 1970s, the prevailing dogma held that macrophages were the predominant antigen-presenting cells. After all, everyone knows that macrophages are the quintessential phagocytic cells that engulf and destroy pathogens and damaged cells. The first Nobel Prize in cellular immunology was awarded in 1908 to Ilya Metchnikoff for his discovery of macrophages and phagocytosis. Not surprisingly, when macrophages ingest pathogens, they do present antigens on their cell surface. But surprisingly, no one had ever shown that macrophages were the authentic antigen-presenting cells that activate T cells and tell them how to respond to foreign invaders.
Dorothy Parker, the American author celebrated for her caustic wit, famously quipped that “you can’t teach an old dogma new tricks.” But Steinman’s research, carried out in dogged fashion over 35 years, did teach the old dogs of immunology a few new tricks: Macrophages are not the major antigen-presenting cells in the body. This distinction belongs to a new type of white blood cells called dendritic cells, which communicate directly with T cells to initiate the immune response. From Steinman’s discovery, we now know why macrophages do not activate T cells. They stay put in their tissue locations, battle the invaders directly, and do not travel to lymph nodes where the virgin T cells reside.
The story of how dendritic cells were discovered began in 1970 when Steinman joined the laboratory of the late Zanvil Cohn at The Rockefeller University. Cohn was a leader in the field of macrophage physiology who studied the immune response to infection. The original goal of Steinman’s project was to learn how antigens provoke antibody production in a major lymphoid organ of mice, the spleen. In the course of this work, Steinman encountered a very minor population of cells in the spleen that exhibited an unusual shape with long, slender projections that resembled the branches of a tree. Since these newfound cells had never previously been described, Steinman studied them intensely and showed that they possessed properties distinct from macrophages. Since it’s a rare opportunity in this day and age for a scientist to name a newly discovered cell, Steinman thought long and hard about his decision. Impressed by the cells’ long slender projections and their graceful movements, his initial instinct was to call them “claudiacytes” in reference to the long arms and legs of his elegant wife, Claudia, who has also been his ballroom-dancing partner for 40 years. But for reasons known only to him, Steinman ended up choosing the less romantic name of “dendritic cells,” derived from the Greek word dendreon for tree.
Over the next 15 years, from 1975 to 1990, Steinman worked out methods for purifying dendritic cells, learned how to grow and expand them in cell culture, and showed that pure dendritic cells (rather than macrophages) are the major antigen-presenting cells that stimulate T cells to divide and differentiate into helper T cells and cytotoxic T cells. In the mid 1990s, he followed up these cell culture studies with a series of elegant experiments in living animals.
A key concept to emerge from Steinman’s in vivo studies is that dendritic cells mature in different ways, depending on the type of pathogen that triggers their maturation. When an antigen-presenting dendritic cell activates a virgin T cell, the T cell needs to know whether the antigen came from a bacteria, a virus, or from a parasite, each of which requires a different type of response in terms of activating T helper cells or T killer cells. The T cells also need to be able to measure the severity of the infection so that the immune system is able to mount a response that is proportional to the danger posed without overreacting inappropriately.
The key to dendritic maturation is a family of receptors on the surface of each dendritic cell that are tuned to recognize virtually every conceivable pattern of structural features contained in the universe of microorganisms that invade the human body. These so-called pattern recognition receptors are the molecular eyes, ears, and nose of dendritic cells. They measure the nature and severity of the microbial invasion and transduce this information into the program of antigen presentation, which is then communicated to the T cells. The late Charles Janeway of Yale played a key role in the discovery of the pattern recognition receptors in the mid-1990s.
Dendritic cell maturation is one of the most ingenious biological systems devised by Nature. It is the perfect way for one component of a complex defense system to convey accurate information about an impending threat to the second component whose job is to eliminate the threat. The central challenge of any intelligence-gathering system — be it our immune system or our national security system — is the problem of “noise,” the fact that useless information is vastly more plentiful than useful information. Too much noise produces fiascos like the government’s repeated warnings on duct tape and code orange. In the immune system, maturation of dendritic cells solves the noise problem.
One of the important clinical spin-offs from Steinman’s research is the development of a new approach to the treatment of cancer, called dendritic cell vaccination. In a typical scenario, tumor cells from a patient are removed from his or her body and incubated in a test tube together with the patient’s own dendritic cells. Once the dendritic cells become loaded with the tumor’s antigens, the mixture is injected back into the patient to prime his or her T cells, which in turn produces a vigorous immune attack on the tumor. In animal studies, dendritic cell vaccines have produced striking results in shrinking tumors, and they are now being tested in more than 70 patient trials involving many types of tumors — melanoma, prostate cancer, lymphoma, and kidney cancer.
Single-handedly, Ralph Steinman opened a new field of biomedical science. For two decades, he and his team were virtually the only scientists in the world who worked on what turns out to be the preeminent initiating step in immunity that governs the action of T cells. In 1993, Scientific American devoted an entire issue to the immune system with articles by 12 of the world’s leading immunologists. Dendritic cells were never mentioned — not even once in 164 pages. Macrophages were still king. Then, around 1997, almost overnight, Steinman’s concepts went from heretical to conventional.
Why were Steinman’s studies neglected, ignored, and sometimes denigrated by the immunological community for 25 years? The power of the century-old macrophage dogma apparently lulled scientists into brushing aside Steinman’s painstaking experimentation, viewing his novel ideas on dendritic cells as some sort of Victorian curiosity not relevant to the Holy Trinity of Immunity — the macrophage, the T cell, and the B cell. Stirred but not deterred, Steinman passionately believed in a different doctrine — the Divine Doctrine of the Dendreon — and his unshakable self-confidence and faith propelled him to a higher immunological truth.
Of the two defense systems that protect us from the hostile world of foreign invaders, our body’s immune system is far superior in effectiveness to our nation’s security system. If the operations of the CIA and FBI were reorganized around the principles of cooperative interaction exemplified by the Steinman doctrine of dendritic cells and T cells, then our national security system might someday rise from the ashes. To paraphrase Leslie Orgel’s second law, Nature’s immune system is smarter than your government’s security system.
Thank you Ralph for your great work and dedication to science.
Acceptance remarks by Ralph M. Steinman

Acceptance remarks, 2007 Lasker Awards Ceremony
It is exhilarating to be recognized for a Lasker Award and to listen to Joe Goldstein’s exceptional summary. I am so excited that I am actually feeling dendritic, wanting to embrace the members of the jury and all my friends and family who are here today.
I became interested in immunology late in medical school, inspired by leaders like MacFarlane Burnet, Peter Medawar, and Zanvil Cohn. I wanted to know how immunity begins, a basic problem so little understood yet so relevant to many diseases. This question led to the discovery of dendritic cells and to a commitment to figure out how these cells work.
Of course, scientists are always focused on the future, and now I realize that to move forward, we need more people like Mary Lasker and Tony Fauci. One reason that I say this is because we need to mobilize support for the kind of research in patients that leads to discovery. The patient often sets the standard for what we still need to know. Let me sketch three examples where research in patients on dendritic cells should make a difference.
Think about the miraculous vaccines that prevent smallpox, polio, and measles. But why do we still lack vaccines to resist AIDS, malaria, and tuberculosis? Real answers, I feel, will be to discover the principles in humans that allow dendritic cells to bring about vaccine immunity.
Consider the progress in cancer to identify the genetic and subsequent changes that drive malignancy. Again, dendritic cells and the immune system provide a distinct and potentially powerful route to therapies that recognize and attack the changes in cancer cells.
Therefore, we need research in humans to address major scientific frontiers. However, there is currently a lack of patient-based research at many major meetings and journals in experimental medicine. This indicates that support for basic patient-based investigation needs to be built, hence the need for more Mary Laskers and Toni Faucis.
Thank you again to the jury for this exciting honor, thanks for the help I have received from many quarters cited in the acknowledgements at your tables, and thanks to the scientific community and its supporters for the exceptional privilege to be a scientist.
